Are you hoping to find 'how to write ionic formula for compounds'? Here you can find questions and answers on this topic.
Table of contents
- How to write ionic formula for compounds in 2021
- Writing formulas for ionic compounds calculator
- Writing ionic formulas worksheet
- Ionic compound formula examples
- In the chemical formula for an ionic compound, which item is written first
- How to do ionic compounds
- How to write ionic compounds
- Formulas for ionic compounds worksheet
How to write ionic formula for compounds in 2021
.PNG) This image demonstrates how to write ionic formula for compounds.
This image demonstrates how to write ionic formula for compounds.
Writing formulas for ionic compounds calculator
.PNG) This image shows Writing formulas for ionic compounds calculator.
This image shows Writing formulas for ionic compounds calculator.
Writing ionic formulas worksheet
.PNG) This image illustrates Writing ionic formulas worksheet.
This image illustrates Writing ionic formulas worksheet.
Ionic compound formula examples
 This image representes Ionic compound formula examples.
This image representes Ionic compound formula examples.
In the chemical formula for an ionic compound, which item is written first
 This picture illustrates In the chemical formula for an ionic compound, which item is written first.
This picture illustrates In the chemical formula for an ionic compound, which item is written first.
How to do ionic compounds
.PNG) This image shows How to do ionic compounds.
This image shows How to do ionic compounds.
How to write ionic compounds
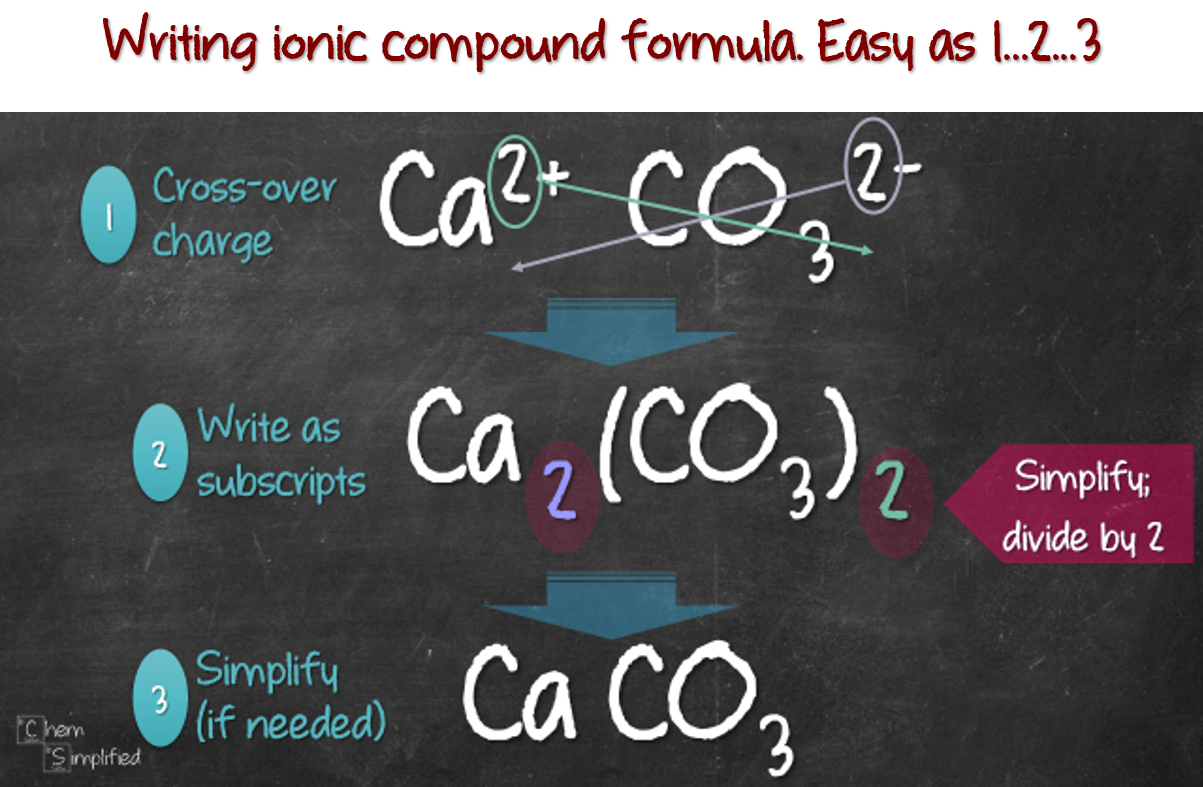 This picture representes How to write ionic compounds.
This picture representes How to write ionic compounds.
Formulas for ionic compounds worksheet
 This picture shows Formulas for ionic compounds worksheet.
This picture shows Formulas for ionic compounds worksheet.
How to write formulae for simple ionic compounds?
So the formula for magnesium chloride is MgCl2. As another example, if you knew that the charge on a sodium ion was +1, Na+, and the charge on an oxide ion was 2-, O2-, then it is easy to see that the formula for sodium oxide is Na2O. You need to have two sodium ions to balance the charges on the oxide ion. Important!
How to write the ionic equation for hydrochloric acid?
Each formula unit of H2SO4 dissociates to give 2 hydrogen ions, H+, and 1 sulfate ion, SO42-. Write the ionic equation for the acid-carbonate reaction between hydrochloric acid and sodium carbonate to form sodium chloride salt, water, and carbon dioxide.
What do you need to know about ionic equations?
The basics of writing ionic equations: knowing your solubility table In ionic equations, we express dissolved ionic compounds as their dissociated ions, like Na + (aq) and Cl – (aq). This means that we have to know what is soluble, and what is not. Compounds of…
Which is the first part of an ionic compound?
Basic compounds do not contain transition metals and contain only 2 ions. Write the name of the metal. The first part of an ionic compound is called the "cation," which is a metal. This is the positively charged ion in the compound, and it is always written first in ionic compound formulas.
Last Update: Oct 2021